

Copyright 2015 © Nigerian Journal of Paediatrics. All Rights Reserved. . Powered by Pelrox Technologies Ltd
ISSN 03 02 4660 AN OFFICIAL JOURNAL OF THE PAEDIATRIC ASSOCIATION OF NIGERIA


Quick Navigation


Niger J Paediatr 2017; 44 (1):26 – 31
ORIGINAL
Adisa AK
Prevalence and pattern of bacte-
Hassan-Hanga F
Oyelami OA
raemia among HIV-infected under
-five children in a tertiary
hospital in Kano, Nigeria
DOI:http://dx.doi.org/10.4314/njp.v44i1.5
Accepted: 6th December 2016
Abstract :
Background: Bacterae-
typhi and Staphylococcus aureus
mia is an invasive bacterial dis-
were the predominant isolates,
Adisa AK (
)
ease of childhood that is associ-
each accounting for 21% of all
Department of Paediatrics,
ated with serious complications
cases
of
bacteraemia.
Most
Aminu Kano Teaching Hospital
and high mortality especially in
(81.3%) of the subjects were on
PMB
3452, Zaria Road
Kano, Nigeria
immunocomprised HIV infected
HAART and its use had no effect
Email: adisakolly@yahoo.com
children.
on
rate of bacteraemia. Fourteen
Aim: To
determine the
prevalence
(73.7%) and 12(63.2%) of the iso-
Hassan-Hanga F
and pattern of bacteraemia among
lates were sensitive to ciproflox-
Department of Pediatrics,
HIV-infected Under-five children.
acin and ceftriaxone respectively.
Aminu Kano Teaching Hospital/
Design: It
was a
prospective cross
Sensitivities to ampicillin, clox-
Bayero University Kano, Kano Nigeria
-sectional study
acillin and co-trimoxazole were
Subjects and Methods: One
hun-
0.0%, 5.3% and 5.3% respectively.
Oyelami OA
dred and thirty four febrile HIV-
Conclusion: Bacteraemia
is a
sig-
Department of Pediatrics and Child
infected children were recruited
nificant health problem among
Health, Obafemi Awolowo University
from the outpatient departments
HIV-infected under-five children
Teaching Hospital Complex, Ile-Ife,
and emergency room of a tertiary
despite the high rate of HAART
Osun State
hospital to determine the presence
use. Treatment adherence should
of
bacteraemia, the etiologic agent
be
strengthened among this popu-
and antibiotics susceptibility. An
lation. There is need for improve-
automated (BACTEC) incubator
ment in personal and food hygiene,
was used to detect bacteraemia,
environmental sanitation and pos-
subcultures were done and identi-
sibly introducing typhoid vaccine
fication and antibiotic susceptibil-
among under-five HIV-infected
ity tests were done using standard
children.
laboratory procedures.
Socio-
demographic and clinical data
Keywords: bacteraemia,
under-
were obtained using a proforma
five, human immunodeficiency
and
data analysis was done using
virus/acquired immune deficiency
SPSS version 17.0 for windows.
syndrome, prevalence, highly ac-
Results: The
prevalence of
bacte-
tive antiretroviral therapy.
raemia in HIV-infected children
was 14.2% (19/134). Salmonella
Introduction
depending on the presence of an underlying turbulent
cardiac flow or immunosuppression as in HIV infection.
Seventy one percent of world’s HIV -infected children
A
form of clinical bacteraemia associated with fever but
live
in Sub-Saharan Africa. Nigeria accounts for an
1
no
evidence of sepsis or clear focus of infection is
termed “occult bacteraemia”. In this report, we shall
6
estimated 10% of global burden of HIV/AIDS and 30%
of
the burden of mother-to-child transmission of HIV
use the term “bacteraemia” to mean all clinically signifi-
with
the national HIV prevalence rate of 3.4%. Over
2,3
cant forms.
440,000 Nigerian children under the age of 15 years are
living with the infection and three out of every 100
In
the HIV-infected, bacteraemia is a serious condition
deaths in children are due to HIV/AIDS directly or indi-
that often persists and could lead to potentially lethal
rectly.
2,4
diseases including pneumonia, septic arthritis, osteo-
myelitis, meningitis and severe sepsis. Factors such as
7
Bacteraemia is the presence of viable bacteria in the
abnormalities in humoral and cell-mediated immunity,
circulating blood. This may or may not be symptomatic
5
phagocytic cell dysfunction and skin and mucous mem-

27
brane defects all contribute to higher risk of bacteraemia
Sampling/data collection
in
them. Bacterial infections are responsible for the
8
immediate cause of death of up to 30% of patients with
By
serial recruitment, every eligible HIV-infected child
HIV infection.
9
who met the inclusion criteria was recruited from the
Unfortunately, 630,000 African children are receiving
PIDC or EPU. Diagnosis of HIV infection was based on
the Nigerian national protocol.
4
Anti-retroviral Therapy (ART) which corresponds to
only 22% of those that are eligible. In Nigeria, the
10
During routine clinic visit and emergency room consul-
situation is even more distressing as only 15% of the
tation, eligible participants were identified, proforma
eligible 92,200 Nigerian HIV-infected children have
administered and blood samples were obtained for blood
access to this life-saving treatment modality.
2
culture and blood counts. A complete physical examina-
There is a paucity of data on bacteraemia in HIV-
tion was also carried out on each child.
infected children particularly in the northern part of the
country. More so, results from few studies from other
Subjects were classified based on the history, clinical
parts of the country such as Benin may not be applica-
11
examination findings and most recently documented
ble to patients in our locality. This study was carried out
CD4 count into the appropriate WHO clinical and im-
to
determine the prevalence and pattern of bacteraemia
munological stages. The Partec Cyflow counter serial
in
HIV-infected children. It is hoped that results of this
number 050117117 was used for the estimation of CD4
study would provide the basis for cost-effective inter-
count and percentages were calculated as a fraction of
ventions such as chemoprophylaxis, vaccinations and
the Total Lymphocyte Count (TLC) obtained from full
rational use of antibiotics that will improve the manage-
blood count of the same sample. The most recent CD4
ment and outcome of children with HIV/AIDS.
percentages obtained during infection free period were
used to classify subjects into different immunologic
categories using the revised WHO staging of 2007.
Subjects and Methods
Sample collection
Study design
In
each case, the procedure was explained to the parent/
caregiver. The site thoroughly cleaned, with 70% isopro-
The
study was a hospital-based prospective cross-
pyl
alcohol solution and tourniquet applied, followed by
sectional study conducted between August 2014 and
povidone iodine solution that was applied in a circular
June 2015.
pattern and then allowed to dry. Two to three milliliters
(2-3ml) of blood was obtained following a sterile proce-
Study population
dure by inserting an appropriate-sized vacutainer needle
into an antecubital vein in the arm or any other site
The study population included children aged between 6
deemed appropriate with the opposite end puncturing
weeks and 60 months, confirmed to have HIV infection
into the vial for direct inoculation. Prior to the inocula-
presenting on follow up to the Pediatric Infectious Dis-
tion, the flip-off cap of the commercially produced vials
containing BD BACTEC
TM
Peds Plus
TM
eases Clinic (PIDC) and the Emergency Pediatrics Unit
media was
(EPU).
wiped with alcohol swab and allowed to dry. An addi-
tional 2mls of blood was put in an EDTA bottle for
Inclusion criteria
blood counts which served as an initial markers of infec-
tion
pending the availability of culture results.
HIV-infected children aged between 6 weeks and 60
months, on follow up at the PIDC and EPU during the
Laboratory methods
study period. Those who were either febrile or hypother-
mic at presentation and whose parents/caregivers con-
Inoculated blood culture vials were delivered to the
sented to the study.
laboratory within one hour of collection for placement in
the incubator. Samples were incubated in the automated
Exclusion criteria
BACTEC 9050 blood-culture system (Becton Dickin-
son, Temse, Belgium) for a maximum of five days.
Those who had antibiotics (other than co-trimoxazole
Whenever there was a positive signal from the incubator
which is routine in under-five HIV-infected children)
(usually within 48 hours), an aliquot was obtained from
within one week prior to enrolment.
the vial with a sterile syringe and needle and further
examined by Gram stain and sub-cultured onto appropri-
Ethical approval
ate
solid media (blood, chocolate and MacConkey agars)
for 48 hours. Vials with no signals after five days of
Ethical approval for the study was obtained from the
incubation in the BACTEC system were checked by
Research and Ethics Committee (REC) of the hospital.
Gram stain and sub-cultured onto solid media for the
A
written informed consent to enroll the patient into the
same duration of 48 hours prior to discarding as nega-
study was obtained from the parent(s) or the accompa-
tive. Blood cultures were considered positive if a defi-
nying caregiver(s) of each child.
nite non-contaminant pathogen was isolated after a
maximum of seven days.
28
For bacterial identification, all positive blood cultures
Table 1: Socio-demographic
characteristics of
the subjects
were examined directly by Gram stain microscopy and
Variable
Frequency(%)
subcultured on standard media plates. Identification of
the organisms was obtained by biochemical and sero-
Age categories (months)
logical tests. Susceptibility to ampicillin, amoxicillin-
0-11
45(33.6)
clavulanate, cefuroxime, ceftazidime, ceftriaxone, co-
12-35
55(41.0)
trimoxazole, ciprofloxacin, cloxacillin, gentamicin, ox-
36-60
34(25.4)
acillin and ofloxacin were tested using the Kirby-Bauer
Mean (SD)
25(16.6)
disc diffusion method. Preliminary results were made
Gender
available to the managing physicians within 48 hours
Male
80(59.7)
Female
54(40.3)
and the final results after subculture and sensitivity in 7-
Ethnic Group
9
days.
Hausa/Fulani
108(80.6)
Yoruba
5(3.7)
Data analysis
Igbo
10(7.5)
Others
11(8.2)
Statistical analysis was conducted using the statistical
Socio-Economic Class
software package, SPSS version 17.0 (Chicago, IL,
Lower
67(50.0)
USA). Data were presented using frequency tables and
Middle
52(38.8)
cross-tabulations. Quantitative variables were summa-
Upper
15(11.2)
Maternal Education
rized using the mean, median, range, interquartile range
Tertiary
6(4.5)
and standard deviation while qualitative variables were
Secondary
15(11.2)
summarized using frequencies and percentages. Chi
Primary
45(33.6)
square ( χ ) test was used to determine significance of
2
No
formal education
68(50.7)
association between age and prevalence of bacteraemia
and Odds ratio (OR) for association between use of
The overall prevalence of bacteraemia was 14.2%
HAART and prevalence of bacteraemia. Confidence
(19/134). The prevalence was 15.6%, 12.7% and 14.7%
level was set at 95% and a p-value of < 0.05 was consid-
for the 0-11months, 12-35months and 36-59months age
ered significant.
groups respectively. There was no statistically signifi-
cant association between age and bacteraemia (Table 2).
The predominant organisms responsible for bacteremia
were Salmonella
typhi and Staphylococcus aureus , each
Results
accounting for 4/19(21%) respectively.
Streptococcus
pneumoniae was
the second
commonest isolate
account-
A
total of 134 febrile HIV-infected were recruited with a
ing for 3 /19(15.8%)(Table 3).
mean age (SD) of 25(16.6) months. There were 80
(59.7%) males and 54(40.3%) females and the male-to-
Table 2: Prevalence
of bacteraemia
according to
age groups
female ratio was 1.5:1. Forty five (33.6%) of the sub-
among HIV-infected children
jects were infants and 108(80.6%) were of the Hausa/
Bacteremia
Fulani ethnicity. Half (50%) of them were from the
Age
Group(mo)
Positive
Negative
lower socio-economic class (Table 1).
One hundred and nine (81.3%) subjects were on
0-11
7(15.6)
38(84.4)
HAART and the highest proportion (32/34) of those on
12-35
7(12.7)
48(87.3)
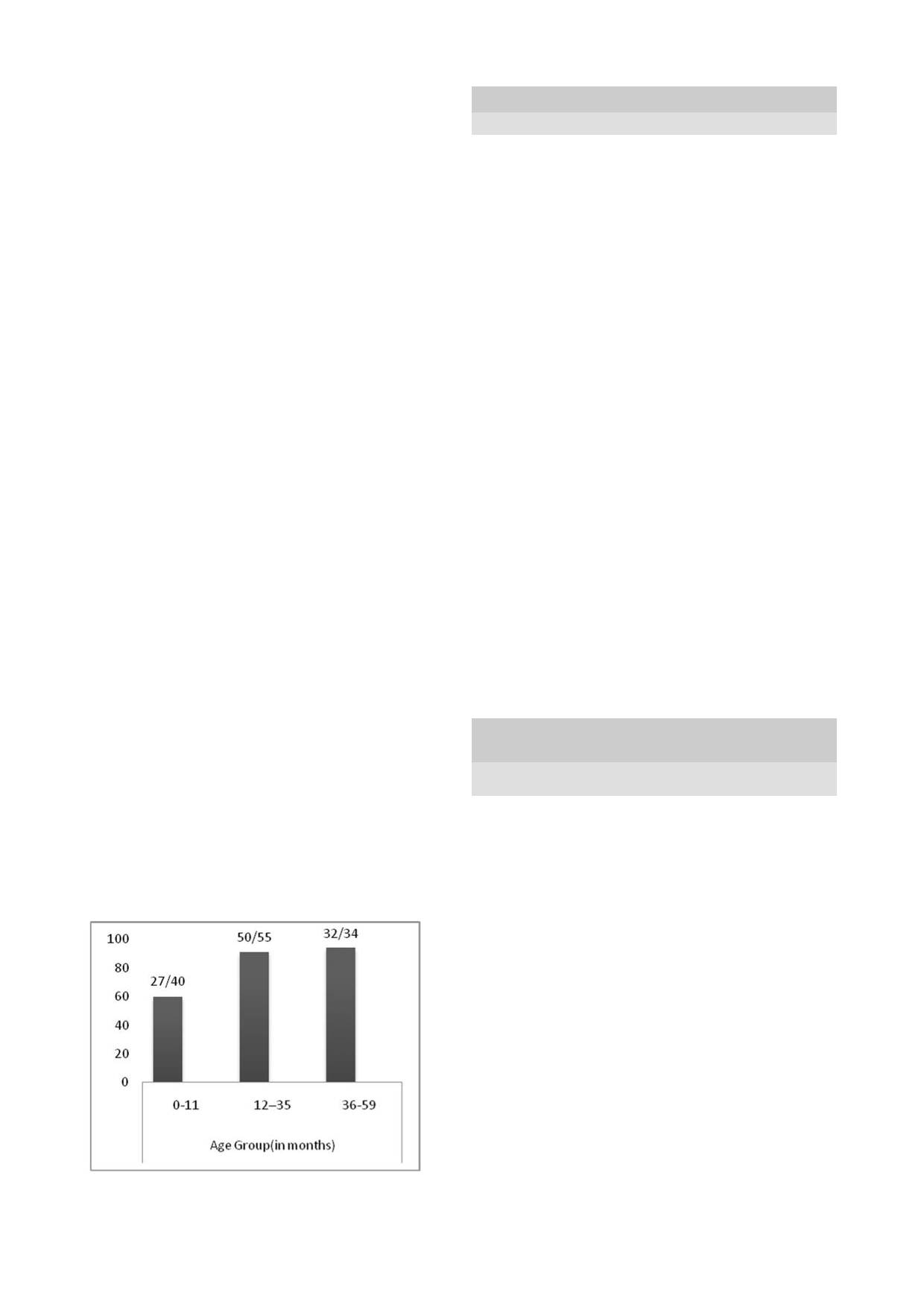
HAART was among the 36-59 month age group (Fig 1).
36-59
5(14.7)
29(85.3)
Total
19(14.2)
115(85.8)
Fig 1: Proportion
of HIV-infected
group on
HAART
χ
=0.17, df=2, p-value = 0.92
2
according to age groups
There was no statistically significant difference in the
prevalence of bacteraemia between those on HAART
and those that were ARV-naïve (Table 4).
Fourteen(73.7%) of the isolates were susceptible to
ciprofloxacin while 12(63.2%) were susceptible to cef-
triaxone. The only NTS isolated was sensitive to co-
trimoxazole, ciprofloxacin and amoxicillin-clavulanate.
Susceptibility to co-trimoxazole was 1(5.3%) and the
overall sensitivity was lowest for ampicillin(0.0%) and
oxacillin(0.0%)(Table 5).

29
Table 3: Etiologic
agents of
bacteraemia in
subjects
Organism
S
typhi
S
aureus
S.
Other K.
Kleb
HIb
NTS
E.coli
Proteus
CoNS
pneumo
spp
pneumo
spp
Total (%)
No
of isolates
4
4
3
2
1
1
1
1
1
1
19
(%)
(21.0)
(21.0)
(15.8)
(10.5)
(5.2)
(5.3)
(5.3)
(5.3)
(5.3)
(5.3)
(100.0)
Organisms: S. typhi-Salmonella typhi, S. aureus-Staphylococcus aureus,
S.pneumoniae - Streptococcus pneumonia, K. pneumo –
Klebsiella
pneumoniae, Hib – Haemophilus influenza type b, NTS-Non-typhoidal Salmonella, K.
spp – Klebsiella species, E. coli-
Escherichia
coli, Proteus spp – Proteus species CoNS- Coagulase-Negative
Staphylococcus.
Table 5: Antibiotic
sensitivity pattern
by isolates
in HIV-infected
subjects
Isolates
No.
CIP
OFL
CFZ
AMP
CXO
CFU
GEN
AUG
CLO
OXA
COT
S.
typhi
4
3
-
-
-
3
3
2
2
-
-
-
S.aureus
4
4
-
1
-
3
-
2
-
1
-
-
S.
Pneumo 3
-
-
3
-
2
1
-
1
-
-
-
Other
2
2
-
2
-
-
-
1
-
-
-
-
Kleb sp
Kleb.
1
1
-
-
-
-
-
1
-
-
-
-
pneumo
Hib
1
-
-
1
-
1
1
-
1
-
-
-
NTS
1
1
-
-
-
-
-
-
1
-
-
1
E.
coli
1
1
-
-
-
1
-
-
1
-
-
-
Proteus
1
1
1
-
-
1
-
-
-
-
-
-
spp
-
-
-
1
-
1
-
-
-
-
CoNS
1
1
Total
19(100.0) 14(73.7)
1(5.3) 7(36.8) -(0.0)
12(63.2) 5(26.3) 7(36.8) 6(31.6)
1(5.3)
0(0.0) 1(5.3)
Isolates:
S.
typhi- Salmonella
typhi , S. aureus-
Staphylococcus aureus , S.pneumo
- Streptococcus
pneumoniae , Kleb. pneumo –Klebsiella
pneumoniae ,
Hib – Haemophilus influenza e,
NTS-Non-typhoidal
Salmonella, Kleb.
spp – Klebsiella species, E.
coli- Escherichia
coli , Proteus
spp – Proteus species, CoNS-
Coagulase-Negative
Staphylococcus.
Antibiotics:CIP- Ciprofloxacin, OFL- Ofloxacin ,CFZ- Ceftazidime, AMP-
Ampicillin, CXO- Ceftriaxone, CFU- Cefuroxime,
GEN- Gentamicin, AUG- Amoxi-clavulanate, CLO- Cloxacillin, OXA-Oxacillin, COT-
Co-trimoxazole.
Table 4: Relationship
between the
use of
HAART and
preva-
positive cultures associated with other methods of cul-
lence of bacteraemia
ture that were used in many of the other studies that re-
HAART
Bacteraemia
ported higher prevalence.
Positive (%)
Negative(%)
Total (%)
The prevalence of bacteraemia was not related to age
Yes
15(13.8)
94(86.2)
109(100.0)
among the HIV-infected subjects. This is not consistent
No
4(16.0)
21(84.0)
25(100.0)
with the fact that the immune system is normally most
Total
19(14.2)
115(85.8)
134(100.0)
naïve at birth and develops over time. Our finding may
be
explained by the presence of HIV infection which is
OR[95% CI]=0.84[0.25, 2.79]
likely to have distorted the trend.
12
A
previous HIV study suggested that apart from the
Discussion
higher burden of bacteraemia in sub-Saharan Africa, the
spectrum of aetiologic agents differ from those in devel-
oped world. Such differences may also be seen within
15
In
this study, the prevalence of bacteraemia in HIV-
infected under-five children was 14.2%. This is similar
developing countries. An increase in bacteraemia due to
to
the report by Madhi et al. with high rate of bacterae-
12
non-typhoidal Salmonella (NTS) and Mycobacteria spe-
mia in HIV infected children of comparable age group.
cies was observed among HIV-infected individuals in a
2010 study in Uganda, but we observed that
Salmo-
16
The prevalence from our study is also similar to that in
the Anti Retroviral Research of Watoto (ARROW)
nella typhi and Staphylococcus
aureus are
the most
pre-
study (14.5%) and also the 15.5% found in a study at
13
dominant causes of bacteraemia in HIV-infected in our
the Kwa-Zulu Natal hospital in South Africa.
14
How-
population. The present study was among under-five
ever, prevalence observed by Imade and colleague in
11
children with poorer hygiene practices (compared to
Benin was much higher(37.1%). This difference may be
adults) while the Ugandan study was among older popu-
due to the fact that most of the subjects in our study
lation in addition to the fact that aerobic culture media
were on HAART and may have been able to reconstitute
used in our study is not suitable for isolating Mycobac-
their immune system thereby having a lower predisposi-
teria. The finding of Salmonella typhi as a common iso-
tion to opportunistic infections. In addition, we found a
late may be explained by the low level of water and food
very low rate of bacteraemia due to Coagulase-Negative
hygiene that characterize most poor and developing so-
cieties like Nigeria . However, this finding is in con-
17
Staphylococcus (CoNS) which is a notable contaminant.
This might have significantly reduced the rate of falsely
trast to Tanzanian studies that reported and postulated
30
12
low risk for Salmonella
typhi among HIV-infected
in separate studies by
Imade et al.
11
and Madhi et al.
population.
18,19
Understandably, Staphylococcus aureus
this was the case.
Furthermore, nearly all the isolates
is
a ubiquitous organism that commonly colonizes the
were resistant to
co-trimoxazole just as Madhi et al.
12
skin and nostril of most individuals regardless of the
reported in Soweto.
This is largely attributable to its
immune status, they become pathogenic when they find
routine use in the HIV
group as prophylaxis against op-
their way into deeper body structures which is easier in
portunistic infections
like Pneumocyctis jiroveci pneu-
HIV infection as a result of defective mucosal barriers.
20
monia (PCP),
toxoplasmosis and malaria.
Our findings agree with reports of Imade
et al. in Be-
11
nin Nigeria and Nchabeleng
et al .
in Kwa-Zulu Natal,
14
South Africa where Staphylococcus
aureus was found to
be the commonest
isolate.
Conclusion
Apart from the Benin
study where they found a high rate
Bacteraemia is an
important cause of morbidity and
of CoNS in the
ARV-naïve HIV-infected subjects,
mortality among
under-five HIV-infected children.
Sal-
CoNS is not a prominent
cause of bacteraemia in HIV-
monella typhi and Staphylococcus
aureus are
important
infected
children.
13,14
We found only one case
(5.3%) of
etiologic agents in
children with HIV infection. The use
CoNS infection in this
study and that was detected in a
of the highly active
antiretroviral therapy is expected to
subject with advanced
immunosuppression. This may be
reduces the rate of
bacteraemia but compliance is more
because they are mainly
opportunistic bacteria that are
important. We recommend
the use of ciprofloxacin or
expected to be more
frequent in the immunosuppressed
ceftriaxone as the
empiric first line antibiotics in any
and those with
prosthetic devices and indwelling cathe-
suspected case of
bacteraemia in under-five HIV-
ters.
[21]
In addition, the
automated culture system is as-
infected children. We
also suggest the need to improve
sociated a low rate of
contamination by organisms such
food and water hygiene
and, in the short-term, consider
as CoNS.
introduction of mass
vaccination against Salmonella
Even though some studies
have report that Mycobacteria
typhi to
help reduce
the rate
of Salmonella typhi
bactere-
plays notable role in
bacteraemia among HIV infected
mia in HIV-infected
children. Ampicillin, oxacillin,
children, our study
cannot corroborate or refute their
cloxacillin and
co-trimoxazole as empiric first-line anti-
findings since we only
utilized aerobic media which is
biotics in the
management of suspected bacteraemia
incapable of isolating
Mycobacteria
should be
discouraged.
The use of HAART
significantly reduces the progres-
Limitation
sion of paediatric
HIV/AIDS.
22,23
Over four-fifths
(81%)
of our HIV-infected
subjects were on HAART which
The
culture media used in this study is not sensitive for
may be another reason for
the lower rate of bacteraemia
detecting mycobacteria and anaerobic bacteria which
when compared to findings
of Imade et al and Madhi et
may
play some role in significant bacteraemia
al. Hospital-based
studies in Africa prior to the advent of
HAART showed that
bacteraemia was three times more
frequent among the
HIV-infected than in the HIV-
Authors’ Contribution
uninfected people and
five times more likely to cause
Adisa AK and Hassan-Hanga F conceptualized the
death.
24-26
study, Adisa AK recruited the subjects and analyzed the
The isolates were
sensitivity to ciprofloxacin and cef-
data.
tazidime and highly
resistant to co-trimoxazole. There
Oyelami OA reviewed the concept and edited the manu-
was significant
sensitivity of Salmonella to
ciproflox-
script.
acin but it was not as
susceptible to amoxicillin-
Conflict of Interest: None
clavulanate and
gentamicin as observed among a Thai
Funding: None
population of
HIV-infected subjects.
27
Until recently,
ciprofloxacin had not
been recommended for use in chil-
dren due to concerns
about its musculoskeletal adverse
effects. Recent studies
have shown that these are mild,
28
Acknowledgement
transient and
reversible.
29
This concern over the
years
may have relatively
preserved ciprofloxacin and other
Our sincere appreciation goes to Prof SK Obaro for his
fluoroquinolones (like
ofloxacin) thereby retaining their
support for the automated blood culture.
efficacy and preventing
the development of resistance.
Ampicillin resistance
was high in our study. Similarly,
Reference
1.
UNAIDS. Global Report: UN-
2.
Nigeria, Federal Ministry of
3.
Nigeria, Federal Ministry of
AIDS Report on the Global AIDS
Health. National guideline for
Health. National human immuno-
epidemic 2015 [Internet].2015
Paediatric HIV and AIDS treat-
deficiency virus and acquired
[updated 2015 Nov; Accessed
ment and care. Federal Ministry of
immune deficiency syndrome and
2015 Aug].
Health. Nigeria 2010.
Reproductive Health Survey 2012
(plus II): Human immunodeficiency
virus Testing. J
HIV Hum
Reprod
2014; 2:15-29.

31
4.
United Nations General Assembly
14.
Nchabeleng M, Yeung S, Escott S,
24.
Gordon MA, Walsh AL,
Special Session (UNGASS) Coun-
Wilkinson D, Sturm AW. (2000).
Chaponda M, Soko D, Mbuwinji,
try
Progress Report [internet].
Bacteraemia in HIV-infected chil-
Molyneux ME, et al. Bacteraemia
Nigeria. NACA/UNAIDS; 2012;
dren in a rural Kwa-zulu Natal
and
mortality among adult medi-
Accessed 2013 Apr 18.
Hospital. Int. Conf. AIDS. July 9-
cal
admissions in Malawi – pre-
5.
Spraycar M, ed.
Stedman's
Medi-
14;
13: abstract No. MoPeB2202.
dominance of non-typhi salmo-
cal Dictionary
.
26th
ed.
Baltimore,
15.
Ward JL, Zangwill KM .
Haemophi-
nellae and Streptococcus pneu-
Md:
Lippincott Williams & Wil-
lus
influenzae .
In: Feigin
RD,
moniae. J
Infect 2001;
42: 44-9.
kins; 1995.
Cherry JD, editors. Textbook of
25. Gilks
CF, Brindle
RJ, Otieno
LS,
6.
Thwaites GE, Edgeworth JD, Ghra-
Pediatric Infectious Diseases. 4
th
Watkins WM, Waiyaki PG, Were
nia-Klotas E, Kirby A, Tilley R,
edition. Philadelphia,
PA:WB
JB,
et al. Life-threatening bacte-
Torok ME et al. Clinical manage-
Saunders; 1998:1464-82.
raemia in HIV-1 seropositive
ment of Staphylococcus
aureus
16.
Grant A, Djomand G, De Cock K.
adults admitted to hospital in
bacteremia. Lancet
Infect Dis
Natural history and spectrum of
Nairobi, Kenya. Lancet
1990;
2011; 11:208-22.
disease in adults with HIV ⁄ AIDS
336:545-9.
7.
Benneth NJ. Bacteremia. Med-
in
Africa. AIDS
1997; 11:S43
– S54.
26.
Vugia DJ, Kiehlbauch JA, Ye-
scape [internet] 2015; [updated
17.
Umeh E, Agbulu C. Distribution
boue K, N’Gbichi, JM, Lacina D,
2015 Jun. 22].Accessed 2016 Mar
pattern of Salmonella typhoidal
Maran M et al. Pathogens and
23.Availablefrom:http://
serotypes in Benue state central
predictors of fatal septicemia
www.emedicinemedscape.com/
Nigeria. Int.
J. Epidemiol
2013; 8
associated with human
article/961169.overview.
(1):5749-61.
immunode ficiency virus infection
8.
Kovacs A, Leaf HL, Simberkoff
18.
Crump JA, Ramadhani HO, Morris-
in
Ivory Coast, West Africa. J
MS.
Bacterial infection s.
Med Clin
sey
AB, Saganda W, Mwako MS,
Infect Dis 1993; 168:564-70.
North Am 1997; 81:319-43.
Yang LY, et al. Invasive bacterial
27.
Srifuengfung S, Chokephaibulkit
9.
Stein M, O'Sullivan P, Wachtel T,
and
fungal infections among hospi-
K.
Bacteraemia and antimicrobial
Fisher A, Mikolich D, Sepe S et al.
talized HIV-infected and HIV-
susceptibility in HIV-infected
Causes of death in persons with
uninfected adults and adolescents
patients at Siriraj hospital.
South-
human immunodeficiency virus
in
northern Tanzania. Clin
Infect
east Asian J Trop Med Public
infection. Am J Med 1992; 93:387-
Dis 2011;52:341 – 8.
Health 2005; 36(2):347-51.
90.
19.
Levine MM, Farag TH. Invasive
28.
World Health Organisation.
10.
WHO. Global Update on HIV treat-
salmonella infections and HIV in
Fluoroquinolone use in children.
ment 2013: Results, Impact and
Northern Tanzania. Clin
Infect Dis
Second Meeting of the Subcom-
Opportunities. WHO report in
2011; 52:349 – 51.
mittee of the Expert Committee
partnership with UNICEF and
20.
Todd JK. Staphylococcus. In:
on
the Selection and Use of Es-
UNAIDS.2013 [Updated 2013
Kleigman RM, Stanton BF, Schor
sential Medicines [internet] 2008.
Nov; Accessed 2014 Aug].
NF,
St. Geme III JW, Behrman
Accessed Nov 2015.
11.
Imade PE, Eghafona NO. Inci-
RE,
editors. Nelson Textbook of
29.
Adefurin A, Sammons H, Jacqz-
Paediatrics. 19 edition, Pennysla-
th
dence of bacteraemia in antiretro-
Aigrain E, Choonara I. Ciproflox-
viral-naive HIV-positive children
vania: W.B Saunders Company,
acin safety in paediatrics. Arch
less than five years of age in Benin
2011:904.
Dis Child 2011; 96:874-
-city, Nigeria. Libyan
J Med
2010;
21.
Henderson KL, Johnson AP, Mul-
880 doi:10.1136/
5:10.
ler-Pebody B, Charlett A, Gilbert
adc.2010.208843
12.
Madhi SA, Petersen K, Madhi A,
R,
Sharland M. The changing aeti-
Khoosal M, Klugman KP. In-
ology of paediatric bacteraemia in
creased Disease Burden and Anti-
England and Wales, 1998-2007. J
biotic Resistance of Bacteria Caus-
Med Microbiol. 2010; 59(2):213-9
.
ing
Severe Community-Acquired
22.
Tumbarello M, Tacconelli E, Do-
Lower Respiratory Tract Infections
nati KG, Citton R, Leone F, Spanu
in
Human Immunodeficiency Vi-
T
et al. HIV-associated bactere-
rus
Type 1-Infected Children. Clin
mia: how it has changed in the
Infect Dis 2000; 31: 170-6.
highly active antiretroviral therapy
13.
Musiime V, Cook A, Bakeera-
(HAART) era. J
Acquir Immune
Kitaka S, Vhembo T, Lutakome J,
Defic Syndr 2000; 23:145-51.
Keishanyu R et al. Bacteraemia,
23.
Manfredi R, Nanetti A, Ferri M,
Causative Agents and Antimicro-
Chiodo F. HIV-associated non-
bial Susceptibility Among HIV-1-
mycobacterial sepsis-bacteraemia,
infected Children on Antiretroviral
before and during the highly active
Therapy in Uganda and Zim-
antiretroviral therapy era. AIDS
babwe. Pediatr
Infect Dis
2013;
1999; 13:1274-76.
32:85-62.







