

Copyright 2015 © Nigerian Journal of Paediatrics. All Rights Reserved. . Powered by Pelrox Technologies Ltd
ISSN 03 02 4660 AN OFFICIAL JOURNAL OF THE PAEDIATRIC ASSOCIATION OF NIGERIA


Quick Navigation


Niger J Paediatr 2017; 44 (2): 44 – 49
ORIGINAL
Farouk ZL
Glucose-6-phosphate dehydro-
Ibrahim M
Ogala WN
genase deficiency; the single most
important cause of neonatal
hyperbilirubinaemia in Kano,
Nigeria
DOI:http://dx.doi.org/10.4314/njp.v44i2.1
Accepted: 10th March 2017
Abstract :
Introduction: Glucose-
proportion (60.6%) of the inborn
6-phosphate dehydrogenase defi-
neonates had G-6-PD deficiency
Farouk ZL (
)
ciency is the most common enzy-
(x
2 = 5.5, p = 0.06).
Jaundice was
Ibrahim M
matic disorder of the red cell and
noticed significantly earlier in the
Department of Paediatrics
an
important risk factor for neona-
G-6-PD deficient neonates (mean
Bayero University Kano/Aminu
tal jaundice.
=
2.0, SD = 1 days) compared to
Kano Teaching Hospital, Kano
Methodology: The
aim of
the
(mean = 2.7, SD = 1.6 days) in the
Nigeria
study was to determine the inci-
sufficient neonates (t = 2.3, p =
Email: faroukzubaida@yahoo.com
dence of G-6-PD deficiency
0.02). Sixteen (16%) neonates de-
among jaundiced neonates, and
veloped kernicterus, of these 10
Ogala WN
describe the associated morbidity
(63%) were G-6-PD deficient. The
Department of Paediatrics Ahmadu
and mortality pattern in them.
mortality rate among G-6-PD defi-
Bello University Teaching Hospital,
A
prospective cross sectional
cient neonates was 15% (7 of 46)
Zaria, Nigeria.
study was conducted and we stud-
twice as much as in the sufficient
ied one hundred consecutive jaun-
neonates 7% (4 of 54). Only six
diced neonates (55 males, 45 fe-
neonates 0.6% ware exposed to
males) presenting at Aminu Kano
naphthalene of whom three were G
Teaching Hospital from between
-6PD deficient. Five babies were
2004 and August 2005. G-6-PD
given traditional medicine two of
activity was assayed by Quantita-
which were G6-PD deficient.
tive spectrophotometric method of
Conclusion: G-6-PD
deficiency is
Kornberg; serum bilirubin and
an
important risk factor for neona-
haemoglobin levels were esti-
tal jaundice. Jaundice appeared
mated by standard techniques.
early in the deficient neonates.
Exposure to possible Icterogenic
There is high incidence of kernic-
agents, clinical features of kernic-
terus and mortality among them.
terus and the outcome were noted.
Low admission weight
signifi-
Results: The
incidence of
G-6-PD
cantly contributed to the mortality.
deficiency was found to be 46%
with male to female ratio of 3:1
Key Words: G-6-PD
deficiency;
(
Χ = 15, p = 0.001). A higher
2
Neonatal Jaundice; Kernicterus
Introduction
In
Southern Nigeria reported incidence was 25 to 34.4%
among neonates with jaundice. About 60% of all full
7,8
The enzyme Glucose-6-Phosphate Dehydrogenase is
term babies and 80% of preterm babies will develop
jaundice. Significant hyperbilirubinaemia is seen in
9
present in all cells of the body. G-6-PD deficiency is a
1
genetic disorder in which the enzyme is inadequate in
10.5% of full term neonates and 25.3% near term in-
fants G-6-PD deficiency predisposes to development
10
quantities or lacking in the red blood cells. It is inherited
as
an X-linked recessive disorder It plays a key role in
of
significant hyperbilirubinaemia in the neonates, with
a
reported relative risk of 3.27.
3,11
the protection of cells against oxidative damage through
In
1994 neonatal jaun-
its role in glutathione metabolism.
dice was identified as one of the serious diseases affect-
It
has been well-documented
2-5
that G-6-PD deficient
ing child health not covered by WHO programs in de-
neonates are more prone to neonatal jaundice than neo-
veloping countries.
12
nates with sufficient enzyme activity. An incidence of
G-6PD deficiency among jaundice neonates was found
The aim of the study was to determine the prevalence of
to
be 12.8% among African Americans neonates, and as
3
G-6-PD deficiency, morbidity and mortality associated
high as 35% among Sephardic Jews, 61% in Ghana.
5
6
with G-6-PD deficiency among jaundiced neonates in

45
our area. There is paucity of data concerning the inci-
2000 version 1.1.2 statistical software. Statistical tests
dence of G-6-PD deficiency and its relationship with
Student’s t test for continuous variables; Fisher’s exact
hyperbilirubinaemia from Kano. This temporal gap
test, Analysis of Variance (ANOVA) and the chi-
along as well as the fact neonatal mortality and morbid-
squared test for discrete variables were employed where
ity has continued to be unacceptably high in Nigeria, the
appropriate. Probability (p) value less than 0.05 were
findings of this study will help in planning preventive
accepted as significant. Data on G-6-PD deficient neo-
measures, and consequently decreasing the burden of
nates were compared to sufficient neonates.
hyperbilirubinaemia. Neonates aged 0 to 28 days with
clinical jaundice
Results
There were 55 (55%) males and 45(45%) females’ neo-
Materials
and methods
nates. Thirty-three (33%) of the babies were delivered at
AKTH and 67 (67%) were referred. Eighty (80%) neo-
The study was prospective descriptive in nature, it was
nates were born at term and twenty (20%) were born
carried at Aminu Kano Teaching Hospital, Kano Nigeria
preterm. Half of the preterm neonates were born at
from between November 2004 to and August 2005.
AKTH.
Clearance was obtained from the ethical committee of
Forty-six of 100 neonates studied had G-6-PD defi-
the hospital. Consent was obtained from the mothers of
ciency, giving an incidence of 46% with 95% confi-
the
neonates. Consecutive neonates presenting with
dence interval of 36% to 56.3%. Of the 46 G-6-PD defi-
jaundice or who developed jaundice while on admission
cient neonates, 35 (76%) were males and 11 (24%) were
at
the special care baby unit were enrolled. A total of
females. The male: female ratio was 3:1. The sex differ-
401 neonates were admitted into the unit during the
ence was statistically significant Table I ( Χ = 15, p =
2
study period, of these 115 (28.7%) had jaundice. Of
0.001).The mean G-6-PD activity in the whole study
these 100 met the inclusion criteria and were enrolled.
population of 100 neonates was 5.04 U/gHb. The mean
Neonates that had blood transfusion or whose parents
G-6-PD enzyme activity in the deficient neonates was
declined consent were excluded.
3.1, SD = 1.0 U/gHb Table I shows Jaundice was no-
For every neonate, prenatal history was obtained; history
ticed significantly earlier in the G-6-PD deficient neo-
suggestive of birth asphyxia, history of the time when
nates compared with the G-6-PD sufficient neonates
jaundice was noticed was also obtained. Exposure to
Inborn neonates were younger at presentation mean age
possible Icterogenic agents, such as naphthalene balls,
of
2.88 ± 2.3 days, range 1-9 days, compared with the
henna, dusting powder. The enrolled neonates were
mean age of 5.6 ± 4.8 days, range 2-21 days in neonates
closely monitored for morbidities like anaemia, haemo-
delivered at other hospitals and at home with the mean
globinuria, seizure, hypertonia, hypotonia, high pitched
age of 5.59 ± 2.9 days, range1-14 days. The difference
cry, windmill like movement of the limbs, retrocolis,
was statistically significant (F = 3.7 P = 0.013).
opistothonus feature s of Bilirubin Induced Neurological
Birth weight was recorded in only 39 neonates (all the
Dysfunction (BIND) Score were noted. The outcome
15
33
AKTH born babies and 6 babies born in other hospi-
discharge home, survival with Acute Bilirubin Encepha-
tals). This is because some jaundiced neonates were not
lopathy ABE were noted. Phototherapy or exchange
seen until they were several days old, and there was no
blood transfusion was carried out according to the proto-
record of their birth weight as they were delivered at
col of the unit.
home or other hospitals. The mean birth weight of the
39
neonates was 2925.4, SD = 752.6 g, the range 900-
Laboratory investigations on all the jaundiced neonates
5100g. The mean admission weight of all the neonates
were as follows; Blood typing (ABO and Rhesus
was 2663.5, SD = 798.0g.
groups) for mothers and the babies, blood cultures when
A
total of 94 of the 100 (94%) neonates were breast-
indicated. Total and direct reacting serum bilirubin (SB)
feeding.
using the modified method of Winsten and Cehelyk
13
Using an auto
analyzer
(Express Plus Chiron/
Table 1 :
Comparison the
mean age
at presentation,
mean age
Diagnostics Bio-Rad® California USA). Complete
at
the onset of jaundice and mean total serum bilirubin of the
blood count with supra-vital staining for reticulocyte
neonates withG-6-PD deficiency compared with neonate with
count was done on each sample. Smears for malarial
sufficient enzyme activity
parasites were done when indicated.
Quantitative spectrophotometric method of Kornberg
14
G-6-PD ACTIVITY
Deficient(n = 46) Sufficient(n = 44)
was
used to assay the G-6-PD activity, with G-6-PDH
Mean
SD
Mean SD
t
p
kit procedure No. 345-UV Trinity biotech Wiclow, Ire-
land. Using Spectrulab spectrometer, Surgifield Mid-
Peak
TSB
227.4 97.6
225.5 108.6 - 0.02 0.92
dlesex London. The cut off point for enzyme deficiency
Age
at presentation 4.6 4.1
4.8
3.9
0.29 0.29
is
any value less than 4.6 U/gHb
(days)
Age
Jaundice
2.0
1.0
2.7
1.6
2.3
0.02
Statistical analysis
Was
noticed (days)
Statistical analysis was done with the aid of the EPI info

46
Table 2 :
Comparison of
the mean
Total serum
bilirubin, Hae-
sufficient neonates t ( t = 0.78, df 98, p = 0.44).
moglobin and Reticulocyte count of the jaundiced neonates. G-
The mean weight at presentation in the neonates that
6-PD deficient compared with G-6-PD sufficient neonates
died was significantly lower (both G-6-PDdeficient and
Mean ± SD
sufficient neonates) than the mean weight at presentation
G-6-PD Status
of
neonates that survive as shown in Table 5 (Anova F=
G-6-PD deficient
G-6-PDsufficient
5.6, p = 0.001).
n=
46
n=
54
p
Parameter
Exposure to Icterogenic substances;
TSB
µmol/l
227.5 ± 96.3
225.5 ± 106
0.90
NS
For the majority of the neonates methylated spirit was
(81.6-517.0)
(87.4-609.9)
applied to the umbilical cord. Application of heat with a
Hb
g/dl
13.25±2.9
12.91±3.03
0.57*
NS
piece cloth or a piece of clay pot was also commonly
(4-18)
(4-18)
used in both groups of neonates. Mentholatum ointment
R etic count
% 2.5
± 2.6
2.6± 2.1
0.70*
NS
was applied to the umbilicus in one G-6-PD deficient
(0.2-15.1)
(0.1-10.0)
baby . Five (5%) of the total 100 neonates in the study
There was no statistically significant difference in the mean
were exposed to naphthalene balls, of which 3 were G-6
TSB, haemoglobin levels, reticulocyte count Table 2
-PD deficient. None of the deficient neonates was ex-
posed to henna dye. Five (5%) of the neonates in the
Table 3: The
mean total
serum bilirubin
and Haemoglobin
study were given traditional medicine. Of the drugs
levels by the age of baby when jaundice was noticed. G-6-PD
known to trigger haemolysis in G-6-PD deficient sub-
deficient compared with G-6-PD sufficient neonates
jects, Chloramphenicol eye ointment was used in one
G-6-PD Status
neonate with enzyme deficiency.
Deficient
Sufficient
Age
TSB
µ/
Hb
g/
TSB
µ/
Hb
g/
Table 4 :
Causes of
jaundice among
the 100
babies in
the study
of
mol
dl
mol
dl
onset
Aetiology
n
%
n=46
n=54
[%]
[%]
G-6-PD deficiency alone
17
17
0-1
7[15]
218.6+_1
14.7±2
5[9.3]
237.3±17
12
Sepsis alone
13
13
59
.3
5
±2.5
ABO
incompatibility alone
6
6
(81-547)
(9-17)
(130-
(9-15)
Weight < 2500g
12
12
547)
G-6-PD deficiency+ weight < 2500g + ABO incompatibility 4
4
2-3
12[6]
166+_54
14.2±2
21
208.5 ±
14.4±
G-6-PD
deficiency weight < 2500g + sepsis
4
4
(89.6-
.3
[38]
77
(87.4
2.0
G-6-PD deficiency + Sepsis
8
8
265.2)
(9-17)
-359.)
(9-18)
G-6-PD deficiency + ABO incompatibility,
8
8
4-7
21
260.7+_9
12.4 ±
18
243.7
12.6
ABO
incompatibility + weight < 2500g
6
6
[46]
8
3.3
[33]
±121
±2
G-6-PD deficiency + weight < 2500g
5
5
(110-609)
(4-18)
(110-
(8-18)
Unknown
17
17
611)
Total
100
100
>7
6[13]
250.3+_1
12.7±3
10
230
±103
10.9
00
.0
[19]
(121-
±3.9
n
denote number of neonates
(100.1-
(8-16)
436)
(4-
394)
15)
F
2.9
1.6
0.3
3.7
Table 5 :
The weight
by the
outcome among
the jaundiced
P
0.04
0.20
0.77
0.17
neonates. G-6-PD deficient compared with the G-6-PD
S
NS
NS
NS
sufficient neonate
Data in parenthesis denote percentage [ ] or range ( ) of values
Outcome
G-6-PD status
recorded where appropriate. n is the total number of neonates
Deficient
Sufficient
Weight (g)
Weight (g)
in
the group. F = test value for ANOVA
n=46
Mean
SD
n=54 Mean
SD
F
P
EBT
No
ABE
29
2768.7 711.0 44
2788.1
689.0 5.6 0.001
Survived
ABE
10
2788.1 689.0
6
3280.0
554.0
Exchange blood transfusion was done in 26% (26 of
ABE/Died 7
2192.0 749.0
4
1560.0
563.9
100) neonates. Proportionally there was higher fre-
quency of EBT 60% (15 of 26) among the G-6-PD defi-
F =
test values for Anova, n = number of patients. ABE =
cient compared to G-6PD sufficient neonates this was
Acute Bilirubin Encephalopathy
not statistically significant. ( c =
1.9, p = 0.16).
2
Other causes of jaundice singly or in combinations are
A
higher proportion of the G-6-PD deficient neonates
shown in table V. Two neonates had cephalohaematoma
21% (10 of 46) had clinical features of ABE In contrast,
in
association with G-6-PD deficiency. And 20 neonates
11.1% (4 of 54) of G-6-PD sufficient neonates devel-
were preterm; of these 7 have G-6-PD deficiency. Clini-
oped ABE (c = 2.1, p = 0.15) as shown in figure1.
2
cal diagnosis of sepsis was made in 25 neonates, of
A
total of 11 neonates died giving an overall mortality
these 12 had G-6-PD deficiency. Only two (8%) had
rate of 11%. Five (45.5%) of these were preterm with
bacteriologically proven sepsis. In one G-6-PD deficient
low weigh. The mortality rate among the G-6-PD defi-
baby Staphylococcus
aureus was grown, and
Proteus
cient neonates was proportionately higher 15.2% (7 of
was
grown in another baby with no G-6-PD deficiency.
46
neonates) than the mortality of 7.4% (4 of 54) in the
The
mean duration of hospital stay was 6.38, SD 5.5
G-6-PD sufficient neonates. All the G-6-PD sufficient
days. There was no statistically significant difference the
neonates that died were preterm low birth weight.
length of hospitalization between G-6-PD deficient and
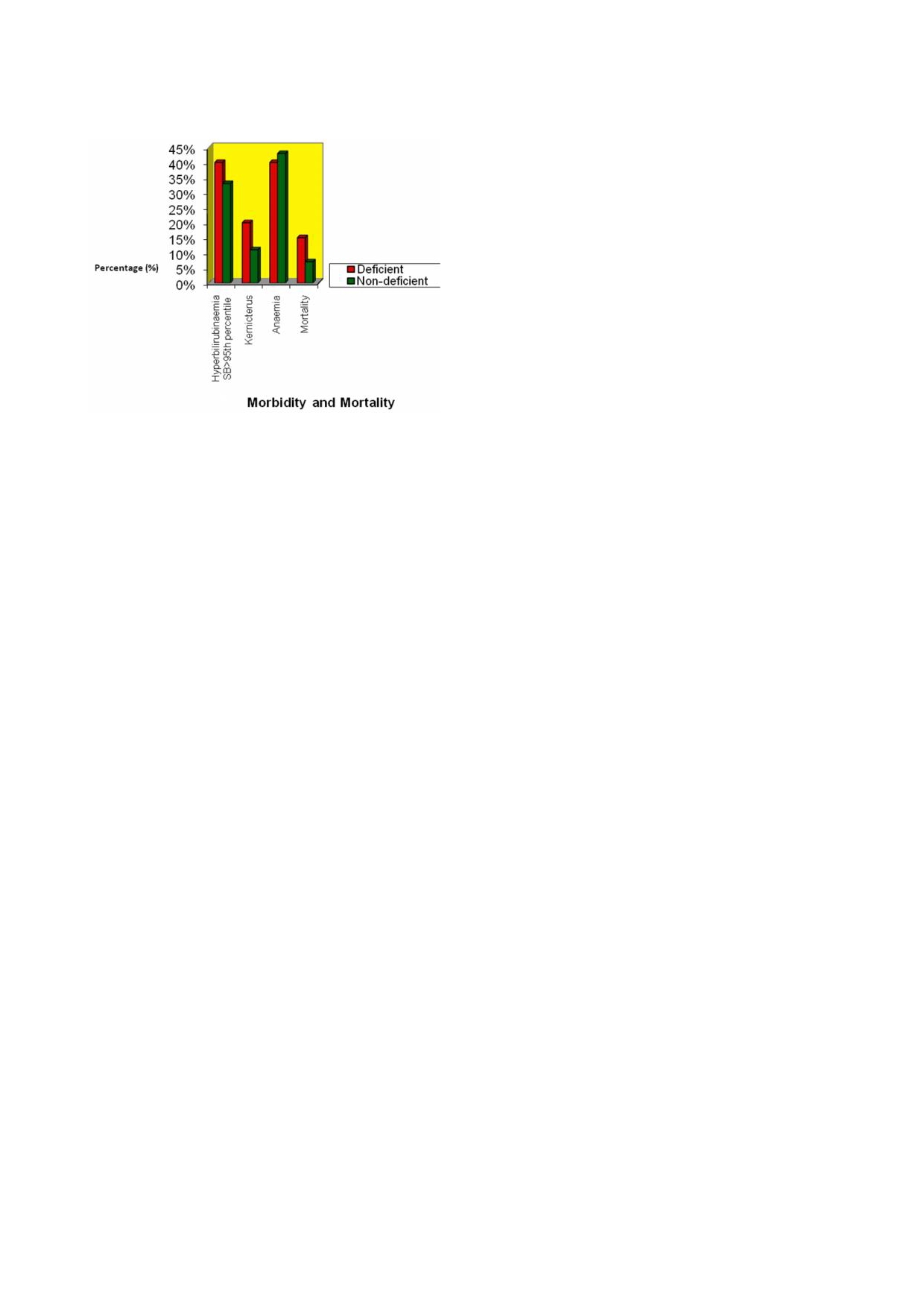
47
Fig 1: Morbidity
and mortality
pattern among
the jaundiced
those of G-6-PD deficient neonates. Other workers have
neonates:G-6-PD deficient compared with G-6-PD sufficient
reported similar findings.
18
On
the other hand, Slusher
neonates
and
co- worker’s reported significantly lower values of
8
haematocrit in jaundiced G-6-PD deficient babies com-
pared with G-6-PD sufficient neonates. They concluded
that haemolysis was the cause of hyperbilirubinaemia in
the G-6-PD deficient neonate.
The frequency of G-6-PD deficiency was proportion-
ately higher among the jaundiced neonates inborn com-
pared with the out born babies. This finding is similar to
what was reported by other workers from Zaria Expo-
15
sureto yet to be identified substances e.g cleaning
chemicals in all babies born at AKTH might have con-
tributed to the jaundice in the G-6-PD deficient neo-
nates. The AKTH babies constitute a ‘homogeneous’
group
or cohort. The out born neonates constituted a
wider group with a less well-defined denominator. The
babies would have been taken to any other health facility
or
may have indeed been left at home. These factors
would conceivably reduce the number of out-born ba-
Discussion
bies presenting to AKTH.
Nevertheless, significant hyperbilirubinaemia in the pre-
The incidence of G-6-PD deficiency in the present study
sent study was observed among relatively higher num-
was found to be high in the order of 46% is in keeping
ber of the out born neonates compared with the inborn
with previous reports from Nigeria 34%, 25%, 62.1%
neonates, in agreement with previous reports from
7,8,11
Zaria Late detection of jaundice and delay at presenta-
15
The male to female ratio of 3:1 in the present study
is
similar to what was previously reported from USA,
tion to hospital observed in the out-born babies might
3,4,5
Israel and Middle East
It
is in keeping with what
have contributed to the greater severity of jaundice in
would be expected from the gene distribution, and the X
these neonates.
linked mode of inheritance of the enzyme deficiency.
The mean TSB in the G-6-PD deficient babies was simi-
The jaundiced inborn neonates were significantly
lar to the mean TSB in the G-6-PD sufficient neonates.
younger at presentation, compared with the out born
This finding is similar to the findings of Kaplan and co-
neonates (P = 0.013). This could be ascribed to a higher
workers, who demonstrated poor correlation between
level of vigilance for jaundice by trained hospital care-
quantitative values of enzyme activity and peak TSB
givers at AKTH, compared with staff of basic or secon-
values. Jaundice in the G-6-PD deficient neonates in the
dary health care facilities and mothers at home.
present study was noticed earlier than in those neonates
There was a high frequency of Acute Bilirubin Encepha-
with sufficient enzyme activity (p = 0.02). This finding
lopathy in the present study, 16% (16 of 100). Glucose-6
is
in keeping what has been reported previously. There
-phosphate dehydrogenase deficiency was found in 63%
is
some evidence that jaundice in the G-6-PD deficient
of
the kernicteric babies (10 of 16). . This is similar to
neonates may have its origin in-utero. However, the age
16
what was reported by other workers in developing coun-
tries.
4,6,7,8
of
presentation was similar between G-6-PD deficient
Even in developed countries a resurgence of
and sufficient neonates. This may suggest a significant
kernicterus is being observed in places where ABE was
previously less frequently observed.
19-21
delay (average of 2 days) from the time jaundice was
noticed and the time of presentation in these babies. This
could be the reason why greater proportion (32%) of the
A
combination of late presentation and genetic predispo-
G-6-PD deficient babies in the study had EBT, also
sition (G-6PD deficiency)appear to play a great role in
21.1% developed kernicterus compared with 20% and
the high incidence of ABE observed. The present prac-
11% of the sufficient respectively.
tice of early discharge of sufficient neonates from our
hospital after delivery (within 24 hour of birth) might
The mean Hb level in the G-6-PD deficient neonate in
have contributed to late identification of jaundice in
the study was slightly low but comparable to what was
some of the neonates with consequent hyperbilirubinae-
found in the sufficient neonates. Similarly the mean re-
mia and kernicterus. The late recognition of jaundice by
ticulocyte count in the G-6-PD deficient was similar to
mother’s and/ or health care providers and delay in re-
that
of the sufficient neonates. These findings may im-
ferral
of jaundiced neonates to tertiary health centers
ply that G-6-PD deficiency does not show association
might have contributed to the higher frequency of ker-
with decrease in Hb or reticulocyte count This could be
nicterus in the present study.
because the G-6-PD sufficient neonates might be experi-
Exchange blood transfusion was done proportionately
encing haemolysis from other causes like blood group
more often in the G-6-PD deficient neonates than in
incompatibility, sepsis and so on. Therefore, their hae-
sufficient neonates. The mean TSB in the neonates that
moglobin and reticulocyte responses would be similar to
had EBT was higher than the mean TSB in the neonates

48
with no EBT (Anova F= 32 p = 0.001) and in line with
may be a reflection of low standard of living and poor
what was previously reported. In keeping with the fact
7
antenatal care in the environment. The setting for ABO
that severe hyperbilirubinaemia was the main indication
incompatibility was present in about a quarter of the
for EBT in the neonates.
neonates.
Sepsis did not seem to contribute significantly to hyper-
bilirubinaemia in the study
.
The high mortality rate of 15% (7 of 46) among G-6-PD
Only
2 neonates had bacte-
deficient jaundiced newborns in the present study is
riologically proven sepsis. This could be because a high
similar to previous reports
6,
8
Late
identification of jaun-
proportion of the neonates were delivered at AKTH
dice
and delay in presentation to the hospital observed in
where standard antiseptic procedures are available,
the
present study could account for this pattern of mor-
which significantly reduced the likelihood of sepsis
tality. Preterm neonates contributed significantly to the
among them. And the out born neonates might have had
mortality. Low weight on admission significantly con-
antibiotics before presentation, which might affect bac-
tributed to the mortality in both the G-6-PD deficient
terial culture. This finding agrees with findings of other
and
sufficient neonates. This is because of the relatively
researchers that infection does not play a significant role
high
number of premature neonate in the mortality.
in
the hyperbilirubinaemia associated with G-6-PD defi-
ciency.
23,
24
Exposure to naphthalene balls and Menthol containing
balm and powder among the G-6-PD deficient neonates
in
the present study was not common and was seen only
The most common factor associated with jaundice in the
in
8.7% (4 of 46). In contrast, reports from southern
present study was G-6-PD deficiency, which was associ-
part of Nigeria found exposure rate to Icterogenic agents
ated with early onset of jaundice, and relative delay of
to
be as high as 73.5%, and thus clearly demonstrated
presentation to the hospital. There was a higher fre-
the association between exposure to Icterogenic agents
quency of kernicterus among the G-6-PD deficient neo-
and jaundice in the G-6-PD deficient neonate.
22
The
nates, and the neonates with G-6-PD deficiency had a
observed variation in the exposure rate could be ascribed
higher mortality rate.
to
cultural differences in the care of the newborn be-
The findings of the present study are indications that G-
tween Kano in the north and the southern parts of the
6-PD deficiency was an important factor in jaundice
country. It is probable that naphthalene-containing sub-
related morbidity, and it contributes significantly to the
stances were not commonly used in households in Kano.
cost of management of jaundice in the study.
Only two neonates were exposed to Mentholatum in the
present study, (one dusting powder, one mentholated
balm), both had G-6-PD deficiency.
Conclusion
Henna dye was applied to one baby only, who had suffi-
cient enzyme activity. Even though women in the north-
There is a high incidence of Glucose – 6-phosphate dehy-
ern
part of Nigeria use henna for skin decoration, it ap-
drogenase
deficiency among jaundiced neonates in
pears that its use in the newborn is not common.
Kano, and it was the single most important aetiological
It
was difficult to evaluate the role of breast-feeding in
factor with respect to neonatal jaundice.
the etiology of jaundice in the present study, as 94% of
It
is recommended that neonatal screening for G-6-PD
all the babies were breast-feeding at the time of presen-
deficiency should be done with the use of hour specific
tation. If breast-feeding contributed to neonatal jaundice
normogram to monitor the rate of rise of TSB in the
it
is possible that it did so in association with other fac-
deficient babies There is a need for public heath cam-
tors in these neonates.
paign on neonatal jaundice and G-6-PD deficiency.
Other common causes of jaundice in the study included,
low admission weight being the second most frequent
cause of jaundice, similar to a previous report. And
Conflict of interest: None
twenty percent of the babies were born preterm. This
Funding: None
References
1.
Glader BE, Lukens JN. Glu-
2.
Mois B, Naisir A, Khan SA,
4.
Nair KA, AL Khusaiby MS.
cose-6-phosphate dehydro-
Amin S, Qhadir KM. Neonatal
Kernicterus and G6PD defi-
genase deficiency and related
hyperbilirubinaemia in infants
ciency – a case series from
disorders of hexose mono
with G-6PD C.563D> T Vari-
Oman. J
Trop Pediatr 2003;
phosphate shunt and glu-
ant. Pediatrics 2012; 12: 126
49(2). Available at: http://
tathione metabolism. In: Lee
3.
Kaplan M, Herschel M, Ham-
www3.oup.co.uk/tropej/hdb/
RG, Foerster J, Lukens J,
merman C, Hoyer JD, Stevens
volume_49/issue_02/
Paraskevas F, Greer PJ, Rod-
KD. Hyperbilirubinaemia
fmg.abs.html/74-774.
gers MG (eds). Wintrobe s
among
African American Glu-
5 .
Kaplan M Abramov
A. Neonatal
Clinical Haematology, 10 ed.
th
cose-6-phosphate dehydro-
hyperbilirubinaemia associated
Philadelphia: Lippincott, Wil-
genase deficient neonates. Pe-
with G6PD deficiency in
liams and Wilkins, 1999: 1176-
diatrics 2004;
114: E
213-E19
Sephardic- Jewish neonates: Inci-
9
Available at: pedialink. Org
dence, severity and the effect of
phototherapy. Pediatrics 1992;
90:402-405.

49
6.
Nkrumah FR. Severe neonatal
12. World health organization. Se-
19. Manning D, Todd P, Maxwell
jaundice. Analysis of possible
rious childhood diseases: prior-
M,
Jane Platt M Prospective
associated factors in neonates
ity issues and possible action at
surveillance study of severe
from Accra. Ghana
Med J
family, community and health
hyperbilirubinaemia in the
1973; 160-5.
center levels. Report of an in-
newborn in UK and Ireland.
7.
Owa TA, Ogunlesi TA. Why
formal consultation. Geneva
Arch Dis Child fetal Neonatal
we
are still doing so many ex-
1984; 9-11.
Ed 2007; 92; f342 – f346
change blood transfusions for
13. Winsten S, Cehelyk B. A rapid
20. Kaplan M. Hammerman C.
neonatal jaundice in Nigeria.
micro diazo technique for
Commentary, Neonatal jaun-
W Journal of pediatr 2009;
measuring total bilirubin. Clin
dice and kernicterus. Pediat-
5:51-55
Clin Chem 1969; 25: 441-6
rics 2001; 108: 263-5.
8.
Slusher TM, Hendrik J,
14. Kornberg A, Horecker BL.
21. Johnson L, Bhutani VK, Karp
McLaren, Lewison JL, Brown
Glucose-6-phosphate dehydro-
K,
Sieveri EM, Shapiro SM
KA, Stevenson KD. Glucose-6-
genase. In: Colowick SP, Kap-
Clinical report from pilot Ker-
phosphate dehydrogenase defi-
lan
NO (eds). Methods in enzy-
nicterus Registry 1992 to
ciency and carboxyhaemoglo-
mology. New York: Academic
2004. J
perinatal 2009
feb 29
bin
concentrations associated
press (publishers), 1955: 323
suppl: S25-S45
with bilirubin-related morbidity
(Quoted by Biotec)
22. Owa JA. Relationship be-
and death in Nigerian neonates.
15. Ahmed H, Hendrickse RG,
tween Exposure to Icterogenic
J Pediatr 1995; 126: 102-108.
Maxwell SM, Yakubu AM.
agents Glucose-6-phosphate
9.
Stoll JB, Kliegman MR. Diges-
Neonatal jaundice with refer-
Dehydrogenase Deficiency
tive system disorders. In. Behr-
ence to aflatoxins. An etiologi-
and Neonatal Jaundice. Acta
man RE, Kliegman RM, Jenson
cal study in Zaria, Northern
Paediatr Scand 1989; 78: 843
HB
(eds). Nelson Text Book of
Nigeria. Ann
Trop Paedi-
-852.
Pediatrics, 17 ed. Philadel-
th
atr.1995; 15: 11-20.
23. Dawodu AH, Owa JA, Fa-
phia: WB
Saunders, 2004:
1635
16. Kaplan M, Algur N, Hammer-
milusi JB. A prospective study
-40.
man C. Onset of jaundice in
of
the role of bacterial sepsis
10. Omit SS, Mahitta A, Korkmaz
Glucose-6-phosphate dehydro-
and G-6-PD deficiency in
A,
Erdem G, Oran O, Yigit S et
genase deficient neonates. Pe-
severe neonatal jaundice in
al
Incidence Course and Pre-
diatrics 2001; 108: 956-9.
Nigeria. Trop
Geogr Med
diction of hyperbilirubinaemia
17. Kaplan M, Vreman HJ, Ham-
1984; 36: 127-32.
in
near term and term new-
merman C et al. Contribution
24. Iskander I, Gamaleldin R, El
borns. Pediatrics
2004; 113:
of
Haemolysis to Jaundice in-
Houchi S, El Shenawy A,
775-78
Sephardic Jewish G-6-PD Defi-
Seoud I, El Gharbawi N,
11. Kaplan M. Hammerman C.
cient neonates. Br
J Haematol.
Youssef H, Aravkin A,
Glucose -6-phosphate dehydro-
1996; 93: 822-827.
Wennberg
R P. Serum
genase deficiency and Severe
18. Bartman p, Schaff F Kernic-
Bilirubin and Bilirubin/
neonatal Hyperbilirubinaemia a
terus in Germany 2003-2005
Albumin Ratio as Predictors
complexity interactions be-
pediatric Academic societies
of
Bilirubin Encephalopathy
tween
Gene and environ-
EPAS 2007; 617936.24
Pediatrics ; 2014 134;e1330-9
ment semin
in fetal
neonatal
2010; 15 148-156.







